When A Chemical Reaction Takes Place In An Open System
When a chemical reaction takes place in an open system. In a chemical equation numbers often appear in front of a chemical fromula. An open system may appear to violate conservation laws because it can gain or lose matter and energy. What happen if a chemical reaction takes place in an open system.
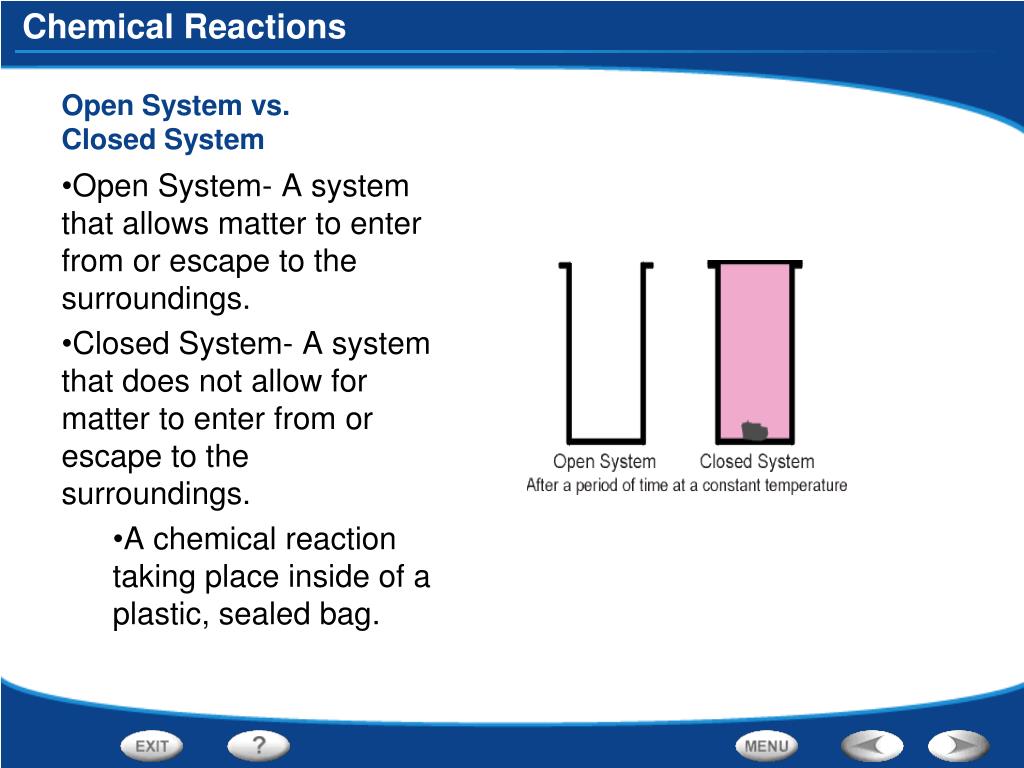
Matter can enter from the surroundings but cannot escape to the surroundings. Matter can enter from the surroundings but cannot escape to the surroundings. A can enter from the surroundings but cannot escape to the surroundings.
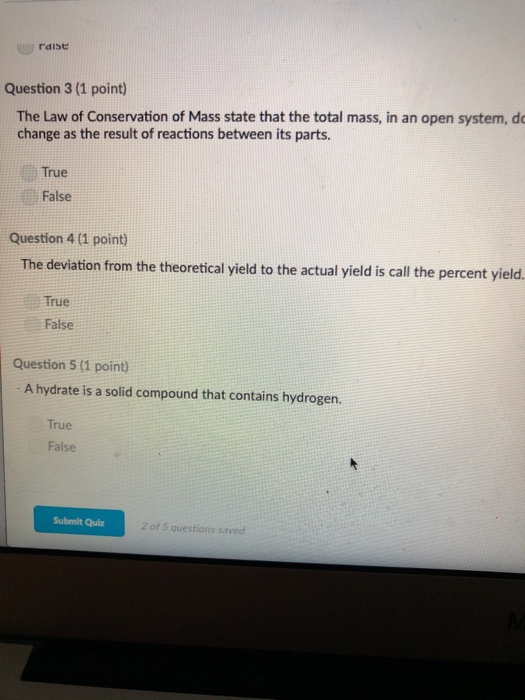
Biological systems or living organisms may also be considered. When a chemical reaction takes place in an open system answer choices. Once the reaction starts the amounts of the reactants will start decreasing and the amounts of the products will begin to increase.
C A chemical reaction takes place in a closed adiabatic perfectly insulated rigid container. Cases Case 1 Case 2 Description A chemical reaction takes place in a closed isothermal reactor and that the temperature in reactor is increased when the reaction was carried out adiabatically A liquid. When a chemical reaction takes place matter is conserved.
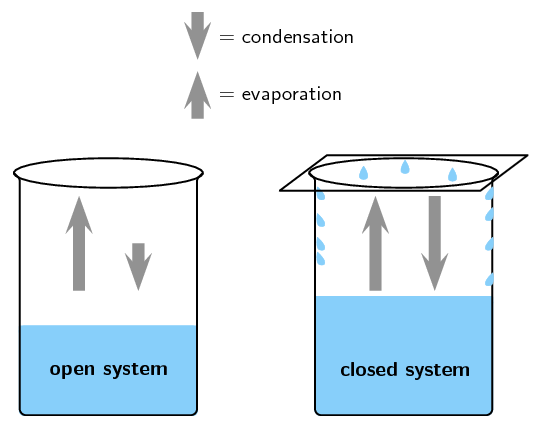
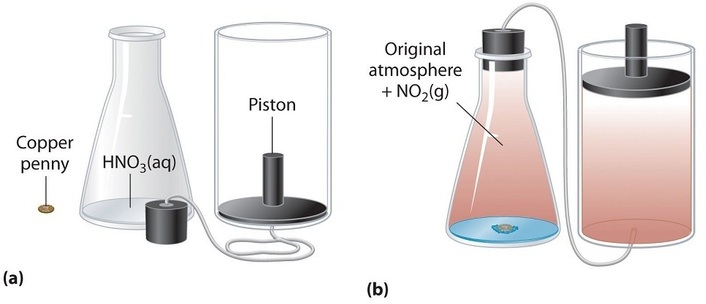
At the instant of mixing you have 1000 mL of a mixture of HCl and NaOH at 220 C. In the liquid-gas demonstration we used the first beaker was an example of an open system because the beaker could be heated or cooled a change in energy and water vapour the matter could evaporate from the beaker. When a chemical reaction takes place in an open system matter can enter from or escape to the surroundings IN a chemical equation numbers often appear in front of a chemical formula.
In science an open system is a system that can freely exchange matter and energy with its surroundings. Such interactions can take the form of information energy mass etc. Matter is not allowed to enter from or escape to the surroundings.
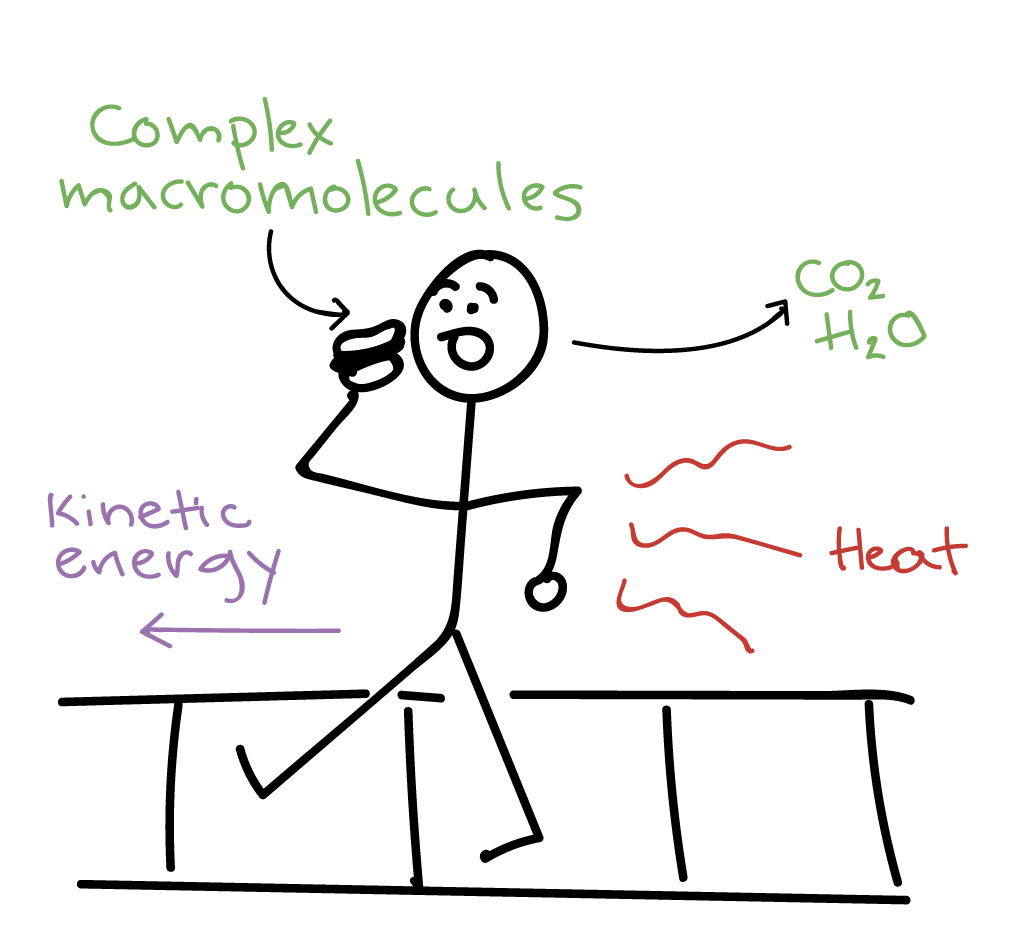
These mass changes allow scientists to monitor rates of reaction the rate of production of a product or. Matter can enter from or escape to the surroundings.
C A chemical reaction takes place in a closed adiabatic perfectly insulated rigid container.
When a chemical reaction takes place in an open system matter can enter from or escape to the surroundings IN a chemical equation numbers often appear in front of a chemical formula. Such interactions can take the form of information energy mass etc. Matter cannot move at all. Matter is not allowed to enter from or escape to the surroundings. In the liquid-gas demonstration we used the first beaker was an example of an open system because the beaker could be heated or cooled a change in energy and water vapour the matter could evaporate from the beaker. Matter is not allowed to enter from or escape to the surroundings. Matter is not allowed to enter from or escape to the surroundings. C A chemical reaction takes place in a closed adiabatic perfectly insulated rigid container. So keeping this definition in mind the law of conservation of mass in open system is difficult because when chemical reaction takes places things change form and some things are released as energy That energy than.
When a chemical reaction takes place in an open system matter can enter from or escape to the surroundings IN a chemical equation numbers often appear in front of a chemical formula. At the instant of mixing you have 1000 mL of a mixture of HCl and NaOH at 220 C. However if a chemical reaction is completed in an open system mass changes may occur. An open system is one in which matter or energy can flow into or out of the system. Zinc reacts with dilute hydrochloric acid to produce zinc. When a chemical reaction takes place in an open system matter can enter from or escape to the surroundings IN a chemical equation numbers often appear in front of a chemical formula. C A chemical reaction takes place in a closed adiabatic perfectly insulated rigid container.



































Post a Comment for "When A Chemical Reaction Takes Place In An Open System"